O’Hara, J.E. 2008. Tachinid flies (Diptera: Tachinidae). Pp. 3675–3686. In: Capinera, J.L., ed., Encyclopedia of Entomology. 2nd Edition. Springer Netherlands, Dordrecht. 4346 pp. [PDF available from author upon request.]
Introduction
There are well over 100 families in the order Diptera, or true flies, and most of them are comprised entirely of species with free-living larvae. There are, however, about 20 families with at least some species classed as parasitoids – species that live within other animals as larvae and kill their hosts before progressing on to the adult stage. It is estimated that this type of parasitic life style evolved over 100 times in the Diptera and multiple times within certain families. About 16,000 of the approximately 120,000 described species of Diptera are parasitoids and about 10,000 of them belong to a single family, the Tachinidae. All tachinid flies are parasitoids in their larval stage and their hosts all belong to the Arthropoda, almost exclusively the Insecta. The true diversity of the Tachinidae is likely many thousands of species higher than the 10,000 currently described, making this family a good candidate for the most speciose family of Diptera and without question the most successful with a parasitic way of life.
Most tachinid flies are larger than a house fly and noticeably more bristly, but they range in size from 2–20mm and across the family there is a tremendous variety of shapes, colors and degree of bristling (Fig. 1). The features upon which the family is founded and that support its monophyly (descent from a common ancestor) are rather subtle. In addition to features shared with other families of the Oestroidea (Rhinophoridae, Sarcophagidae, Calliphoridae, and Oestridae), adult tachinids possess a well developed postscutellum (a rounded bulge below the hind part of the thoracic dorsum) and the labrum (or mouth hook) of the first instar larva is fused with the cephalopharyngeal skeleton. The family is cosmopolitan in distribution and most diverse in the subtropics and tropics.

Classification
The Tachinidae are currently divided into four subfamilies, the Phasiinae, Dexiinae, Exoristinae and Tachininae (see Fig. 1 for representatives of each). The smallest of these in terms of species, the Phasiinae, is possibly but not demonstrably a monophyletic lineage in which all species are parasitoids of Heteroptera, or true bugs. The Phasiinae are morphologically diverse and include most of the tachinid species that lack strong bristling. The Dexiinae are the next largest subfamily and the only one widely regarded as monophyletic, a belief based on shared derived features of the male terminalia. In particular, male dexiines possess a hinged connection between the basiphallus and distiphallus of the phallus (or aedeagus) that is not found in other tachinids. Some of the most visually stunning tachinids belong to the dexiine tribe Rutiliini, an Oriental and Australasian group of mostly large and metallic-colored flies. The Exoristinae and Tachininae are the most speciose subfamilies. The former is more homogeneous in appearance, with many species grayish black in color and moderately bristly. Given this combination of homogeneity and diversity, the Exoristinae are, as a group, the most taxonomically difficult of all Tachinidae. The Tachininae are more morphologically diverse and include some of the largest, most colorful, and most bristly of tachinids. The subfamily also includes some of the smallest and least conspicuous tachinids.
The phylogenetic relationships within the Tachinidae are unclear at the higher levels. There are currently about 40 tribes recognized, but not all are monophyletic and the relationships within and between them are poorly known. Many of the main characters used to distinguish tachinid species are not unique to particular lineages, thus inhibiting attempts to infer relationships through parsimony analysis of external morphological traits. It is possible that more detailed study of male and female terminalia and immature stages will provide better, less homoplastic, characters for phylogenetic analysis. Molecular systematics is beginning to show promise in the elucidation of tachinid relationships, but there have been few studies to date.
The classification at the generic level is relatively stable in the Nearctic and Palearctic regions where the tachinid faunas are fairly well known. There are literally thousands of undescribed species in the other regions, and until those species have been better studied the genera of those regions will remain poorly characterized. Certain authors in the first half of the 1900s had a tendency to oversplit the Tachinidae to the extent that each genus would often comprise only one or two species, and this legacy is still in evidence today in less studied parts of the world. For example, the Neotropical Region has nearly 30% of all described tachinid species but over 50% of all genera.
Life stages
Adult
The variation in size and shape of adult tachinids has been mentioned above and is evident in the specimens shown in Fig.1. Tachinids range in color from pale yellow to jet black, with some species shiny metallic blue or purple (e.g., Gymnocheta), or brilliantly metallic and multicolored (many Rutiliini). Wings are usually clear but are darkened or patterned in some species, or even bicolored. Despite the huge range in size, shape and color within the family, the majority of tachinids are grayish black and 5–10mm in length. Many species have four black longitudinal stripes on the thorax, in contrast to members of the closely related family Sarcophagidae that usually have three stripes.
The head is about the width of the thorax, with compound eyes that vary in size from about half head height to almost head height. Eyes are only rarely holoptic (meeting above) and range from bare to densely haired. Antennae range from tiny to enlarged and in certain species (males only) are bifid, trifid, or elaborately branched (Fig. 2). Mouthparts are present and functional, apically padlike for feeding on liquids (e.g., nectar and honey dew), and occasionally greatly elongated (up to length of body, Fig. 3). Chaetotaxy (arrangement of setae) on the head is greatly varied and very useful for the identification of species.


The thorax is slender to broad, typically setose but almost bare dorsally in many Phasiinae. The prosternum between the fore legs is typically haired in Exoristinae, bare in Phasiinae and Dexiinae, and varied in Tachininae. Legs are setose and in some groups elongated, without elaborate modifications except for the flattened, feather-like setae on the hind tibia of Trichopoda. Wings are well developed, haired along several veins in a few species, and show some variation in venation. Vein M in the center of the wing has a bend near the wing margin (bend rarely absent, e.g., Cinochira) and the vein may terminate in vein R4+5 or the wing margin. Very rarely, vein M fades out at about the usual location of the bend. Chaetotaxy of the thorax, especially the arrangement of setae on the upper surface (i.e., scutum and scutellum), is taxonomically and diagnostically very important.
The abdomen is greatly varied from narrow and long to short and round, and from nearly bare to thickly setose. A few species are very good wasp mimics (e.g., Cylindromyia (Ichneumonops) mirabilis) with a wasp-like abdomen. Males of some Uramya species have the tip of tergite 5 constricted and lengthened posteriorly into an almost cone-like appendage that can be nearly as long as the rest of the abdomen (slightly developed in Uramya halisidotae, Fig. 1). Segments 6 to 11 comprise the terminalia and are highly varied morphologically in both sexes. The female terminalia show obvious modifications related to oviposition: short unspecialized ovipositors in most species that lay eggs on foliage, tubular telescopic ovipositors in some species that lay eggs with great precision on their hosts, and piercers of varied shapes and sizes derived from different sternites in different lineages. The terminalia, particularly those of the male, provide some of the best characters for interpreting the phylogenetic relationships within the Tachinidae and are helpful for identifications at the species and genus levels.
Egg
Modifications to the female reproductive system, egg, and ovipositor permit tachinids to parasitize hosts in a wide variety of ways. In the primitive condition, an unembryonated egg is deposited directly on a host by means of an extensible ovipositor. The egg is typically thick and convex on its upper surface and thinner and flatter on its under surface, and is attached to a host with a glue-like substance. Tachinids employing this mode of attack are termed oviparous because the egg is undeveloped when laid and the first instar is not fully formed for several days. The first instar may then burrow directly through the underside of the egg and through the host’s integument, or may exit the egg through an operculum and then burrow into the host. Certain oviparous tachinids, including most Phasiinae, inject an egg directly into a host by means of a piercing ovipositor. Oviparous tachinids comprise the Phasiinae and some Exoristinae (Exoristiini, Winthemiini, and a few species of Blondeliini and Eryciini). The female reproductive system of an oviparous tachinid is shown in Fig. 4.
The majority of tachinids are ovolarviparous, depositing eggs that contain fully developed first instars. The female reproductive system of these flies is modified to retain fertilized eggs in an ovisac or uterus until they are ready to hatch (Fig. 5). The ovisac stretches and forms a coil as hundreds to thousands of fertilized eggs are added in it. The females of numerous ovolarviparous species still deposit eggs directly on a host, but other strategies, some quite ingenious, are used as well. In most cases the eggs have a thin chorion and hatch almost immediately after deposition. The eggs of many species are laid near a host or are broadcast on its food plant and the motile first instar either crawls to a host or waits for one to pass by. The eggs of Dexiini and most Rutiliini (Dexiinae) are deposited on the ground and the first instar burrows into the soil in search of a beetle host. A few ovolarviparous species have the unusual habit of ovipositing an egg into the mouth of a host. Members of the Goniini (Exoristinae) have tiny “microtype” eggs that they scatter on the food plant of a host, usually in the vicinity of feeding damage, and these eggs hatch only after being ingested by a host. Various types of piercers have evolved in different lineages of ovolarviparous tachinids to inject an egg into a host.
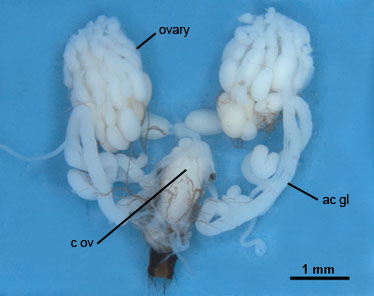
ac gl, accessory gland; c ov, common oviduct.

Some tachinids are cited in the literature as larviparous, meaning in the strict sense of the term that their eggs hatch internally and first instars are oviposited instead of eggs. However, the term larviparous has also been used in a looser sense to describe tachinids that deposit fully developed eggs that hatch immediately after deposition (i.e., ovolarviparous tachinids). The eggs are frequently so thin and membranous as to be almost invisible. In some cases, first instars inside a freshly killed female will break free of their thin-walled eggs and escape through the ovipositor, giving the impression that the species is normally larviparous. Dissections of killed females may also reveal first instars free in the ovisac that would, under normal circumstances, remain in the egg until after oviposition. Although there may be species that are truly larviparous, they have yet to be reliably documented.
The eggs of tachinid flies are not intended to provide long term protection for a first instar. Tachinids do not overwinter in the egg stage or as free-living first instars.
Larval instars
Tachinids, like other cyclorrhaphan flies, have three larval instars. The first instar is remarkably varied externally, adapted to cope with the different circumstances under which eggs are placed in the environment. First instars that must search for hosts on their own have evolved a number of specializations to aid movement and avoid desiccation. Their bodies are often adorned with bands of small spines or sclerotized plates, in some instances displaying more morphological variation between species than the bodies of their adults. First instars of ovolarviparous species can be removed from pinned adult females in museum collections and rehydrated for morphological study. Research into the diversity of first instars is expected to contribute valuable characters for tachinid taxonomy and for phylogenetic analyses of tachinid relationships.
First instars that enter a host on their own do so with the cutting action of a sharp labrum that is varied apically from pointed to axe-like. The labrum is scraped against the host’s integument with a back-and-forth motion until a hole is created, likely aided by an enzymatic substance in the saliva. Once inside the host, the first instar may attach itself posteriorly to its entrance hole or to the host’s tracheal system, or it may remain free in the host’s body. First instars that remain free usually attach themselves to the tracheal system when they become second instars to provide an adequate supply of oxygen for developmental needs. A first instar may start to feed immediately and can pass through all three instars in as little as a few days, but in most species the larval stage lasts a few weeks. Not uncommonly, the first instar delays development until the host reaches a particular stage. It might migrate to specific places within the host during its period of inactivity, such as the salivary glands, ganglia or muscles, where it is able to evade the host’s immune system. It is generally hormonal signals within the host that trigger the first instar to relocate and continue development. Some tachinid species overwinter as first instars within their hosts.
A first instar feeds on host hemolymph and carefully avoids damaging any vital organs, living more like a parasite than a host-killing parasitoid. This changes during the second instar when rapid feeding and growth begin. If a larva did not form a connection to tracheal or outside air during its first instar, then it typically does so as an early second instar. At the same time as the tachinid larva is attempting to feed and obtain oxygen, the host is attempting to kill it through an immune response termed encapsulation. In the early stages of encapsulation, a thin membrane forms around the larva. A first instar can be almost completely encased, but if this happens then it can usually still feed on hemolymph that filters through to it. The larger second instar frees itself anteriorly and begins to feed more aggressively on non-vital tissues, at the same time continuing to stay attached to an air supply posteriorly. The capsule that forms around the larva is termed a respiratory funnel and it becomes larger and thicker as time passes. The ability of the Tachinidae to thwart the encapsulation process is one of the keys to their success. This ability does not afford total protection, and some larvae are killed by encapsulation even in suitable hosts. Other potential hosts that are seldom successfully parasitized may have immune systems that are more effective at combating tachinid invaders or there may be other incompatibilities between parasitoid and host.
The primary cutting structure in the first instar, the labrum, is replaced in latter instars by paired mandibles. The second instar is a voracious feeder that selectively consumes non-vital tissues, especially fat stores. The host may appear normal or somewhat lethargic, or may display atypical behavior induced in some way by the parasitoid. The third instar leaves the respiratory funnel and feeds less discriminately. Except in rare cases, the host is killed and the third instar continues to feed until fully grown or until the food supply is exhausted. The latter situation is not uncommon among tachinids that develop gregariously within a single host and the tachinid adults that develop from under-fed third instars can be half or less the size of typical adults.

Puparium
Pupation in tachinid flies takes place within a puparium, the hardened shell of the third instar. Most commonly the third instar crawls away from the host and pupariates in the soil or ground litter, but in some species pupariation takes place within the host. A few species that pupariate within adult beetles strategically cut openings in the host’s integument to facilitate respiration and the eventual exit of the adult (Fig. 6). Quite a few tachinids overwinter in the pupal stage.
The puparium is roughly cylindrical with two small anterior spiracles and generally two conspicuous and darkened posterior spiracular discs, for respiration. The posterior spiracular discs can be flush with the surface of the puparium or raised above it, and directly level with the midline or above or below it. In a few species the spiracular discs are fused into one. Quite often there are three or four spiracular slits on the upper surface of each spiracular disc. The morphology of the spiracular discs is highly varied and provides useful characters for the identification of species. An adult tachinid emerges from its puparium by inflating a balloon-like ptilinum from behind its face to force open the anterior end of the puparium along a circular line of weakness.
Figure 6. Puparium of Myiopharus neilli within remains of its host, an adult sunflower beetle (Zygogramma exclamationis). Anterior and posterior ends of puparium are visible. The beetle is typically decapitated between the prothorax and mesothorax. Underside of beetle is shown with legs removed. Abbreviations: a spr, anterior spiracle; p spr ds, posterior spiracular disc.
Distribution & evolution
The Tachinidae are well distributed throughout all zoogeographic regions of the world (Table 1), although poorly represented on smaller islands that are distant from larger land masses (e.g., Hawaiian Islands, Galapagos Islands). The total number of described species is about 10,000, but this number is misleading because only the Nearctic and Palearctic faunas are relatively well known. The Neotropical Region has the largest number of described species at about 3000, almost twice that of any other region and representing 30% of the world’s fauna, yet there are so many undescribed species and the region is so vast and under-collected that the actual number is probably well over 5000. Similarly, Australia has a described fauna numbering about 500 species but the actual fauna has been estimated at 3500–4000 species. The tachinid faunas of the Afrotropical and Oriental regions have not been well studied and also contain many undescribed species. Given these circumstances, it is likely that the total number of tachinid species in the world is in excess of 15,000.
| Biogeographic region | Genera | Described species |
| Neotropical | 823 | 2864 |
| Nearctic | 304 | 1345 |
| Palearctic | 405 | >1600 |
| Afrotropical | 213 | 1006 |
| Oriental | 261 | 725 |
| Australasian | 228 | 808 |
| World | 1521 | about 10,000 |
Numbers for genera follow O’Hara (2008) and for described species follow the most recent regional catalogues. Actual number of tachinid species (described + undescribed) in the world is likely greater than 15,000. Some species and many genera are found in more than one region so the values for the world are not the sums of the regional numbers.
Some older insect groups show an interesting pattern of distribution that links the faunal elements in South America, Australia and sometimes southern Africa. These relationships are thought to date back to Gondwanaland when the southern continents were fused into a single landmass. By the end of the Cretaceous Period about 65 million years ago, Africa was drifting northward and the connection between South America and Australia (via Antarctica) was becoming cooler and more tenuous. If the Tachinidae began their radiation in the Cretaceous then evidence of this might be sought in the fossil record and modern distribution.patterns. However, no fossil Tachinidae are known from the Cretaceous and there is no clear evidence of southern relationships, so the family is assumed to be of Tertiary origin.
The sister group to the Tachinidae has not been determined but is to be found among the families Sarcophagidae, Calliphoridae, Rhinophoridae and Oestridae. These five families are united by their possession of a row of setae on the meron (a sclerite above the hind leg) and together comprise the superfamily Oestroidea.
Hosts
The evolutionary success of the Tachinidae is closely tied to their exploitation of arthropods as a source of hosts. The family is a difficult one taxonomically and a relatively young one, suggesting that it is still undergoing rapid radiation within its specialized niche. The life of an endoparasitoid is a complex one with strong selective pressures centered around finding and parasitizing a host. In some cases selection has produced generalists that attack many species of hosts, in others selection has produced specialists that attack only one or a very few host species.
Hosts of Tachinidae are all arthropods, almost exclusively insects. For many tachinid species the hosts are unknown. The non-insect hosts comprise only a few species in the Chilopoda (centipedes), Scorpiones (scorpions) and Araneae (a single record from a spider). These hosts are attacked by a few species of Tachininae, mostly within the Polideini.
The majority of hosts of tachinids are Lepidoptera, mostly caterpillars that feed on foliage in exposed situations but also others that are concealed, such as leafrollers and stem borers. Lepidoptera represent the main hosts of the two largest tachinid subfamilies, Exoristinae and Tachininae, and are also significantly attacked by the third largest subfamily, the Dexiinae. The smallest subfamily, the Phasiinae, attacks only Heteroptera and there is only one genus outside the Phasiinae (Euthera in the Dexiinae) that also attacks heteropteran bugs.
Larval and adult Coleoptera of about 20 families are attacked by a small number of species in the Exoristinae and Tachininae and by the majority of species in the Dexiinae. The greatest number of beetle hosts belong to the Scarabaeidae, larvae of which are parasitized by nearly all members of the sizable dexiine tribes Dexiini and Rutillini; adult scarabs are parasitized by members of the small dexiine tribe Palpostomatini.
Hymenopteran hosts are chiefly larval Symphyta, many of which are exposed defoliators like larval Lepidoptera. A small number of wasps (Vespoidea) and ants (Formicidae) also serve as hosts. Hymenopteran parasitoids belong to the Exoristinae and Tachininae. The remaining hosts of tachinids are also parasitized by members of these two tachinid subfamilies and include the Orthoptera (crickets, grasshoppers, katydids), Blattaria (cockroaches), Mantodea (mantids), Phasmatodea (leaf and stick insects), Dermaptera (earwigs), Diptera (flies), and Embioptera (webspinners).
Some tachinids are solitary endoparasitoids and will fight one another within a host until a single larva remains alive. Other species, especially those that parasitize larger hosts, are not aggressive towards one another and numerous individuals can successfully develop into adults. If conditions are crowded, then some maggots may die and the rest may produce stunted adults. Multiparasitism, the development of more than one larva within a host, can also result in more than one tachinid species successfully parasitizing a single host.
There are a variety of factors that determine how many host species a tachinid species will parasitize. The adult female plays a large role in host range by deciding where to oviposit. If eggs are laid on or near a host or injected into it, then the host range is determined by the specificity of the ovipositing female and the ability of the larva to develop in the selected host. Females of some species search for a particular microhabitat and once in it (e.g., a particular plant species) will oviposit on different potential host species encountered there. One tachinid species, Compsilura concinnata, has become well known because it is highly indiscriminate in its selection of hosts and its larvae are capable of developing in many different species. The female fly injects an incubated egg into a host and the larva escapes the defenses of its varied hosts by living and feeding in the midgut between the peritrophic membrane and gut wall. Compsilura concinnata has almost 200 recorded hosts in numerous families of Lepidoptera and also parasitizes sawflies and a species of weevil.
Host location
The ancestral mode of host finding in Tachinidae likely involved a series of cues, olfactory and visual, that guided a searching female to the right microhabitat and host plant and then to an exposed host, where perhaps tactile stimuli initiated oviposition behavior. This method of host location is still common, but there are many sophisticated behaviors that have evolved to aid the searching tachinid. For example, oviposition in numerous tachinids does not involve the visual sighting of a host. In the case of Goniini, microtype eggs are deposited on the food plant of a host, usually in response to plant volatiles released at the site of feeding damage, and the eggs do not hatch until ingested by a host. Many other tachinids also ovipost on the food plant of a host or in a specific microhabitat (e.g., those that parasitize litter insects like cockroaches and earwigs), not necessarily within sight of a host, and the first instar locates a host on its own or lies in wait for one to pass by. Certain tachinids (e.g., Lixophaga diatraeae) are stimulated to oviposit near the burrow of a concealed host by the odor of the frass that has accumulated at the entrance. Members of the Dexiini oviposit on the ground, leaving the task of finding a soil-dwelling scarab host to their first instars.
The importance of visual cues varies in different tachinids. Those that search for hosts at night must rely mostly on other sensory cues to find an acceptable place to oviposit. Some tachinids that lay eggs on exposed caterpillars not only select hosts of a particular size, presumably by sight, but may also place the eggs near the front of the host where they cannot be reached by the host’s mandibles. A few species that parasitize adult beetles assist their first instars by ovipositing near natural openings such as the mouth, anus and spiracles. Not uncommonly, females that oviposit on a specific part of a moving host have a telescopic ovipositor that can be brought forward under the body and in front of the head to permit egg placement to be visually guided. Some tachinids attack their hosts on the wing, requiring acute vision as well as maneuverable flight.
In most instances where olfaction is involved, the chemical cues that attract a female tachinid to an oviposition site are the standard odors of a host or host plant, or odors (volatiles) given off by a damaged plant that is being fed upon. More unusual are the cues that attract certain Phasiinae to their hosts; these species use the pheromones of their heteropteran hosts as host-finding kairomones. The antennae of these tachinids are up to ten times more sensitive to the bug pheromones than are those of the bugs.
The vast majority of Tachinidae are incapable of hearing, but members of the tribe Ormiini have evolved an inflated prosternum between the front legs that functions as a tympanal organ. This structure allows these tachinids to locate their orthopteran hosts at night by homing in on their mating calls. So effective are these parasitoids in finding their calling hosts that some hosts have evolved strategies to reduce their susceptibility. The tympanal organs are different in male and female ormiines, with those of the female very receptive to the low frequency calls of hosts and to sounds in the ultrasonic range, whereas males are only sensitive to ultrasonic sound. It has been suggested that the sensitivity of these nocturnally-active flies to high frequency sound helps them avoid predators such as insectivorous bats.
Mate finding
Male and female tachinids that are commonly found in meadows and along roadways, particularly on wildflowers, probably mate in those places; e.g., Phasiinae, larger Tachinini, most Siphonini. There are other tachinids that go to species-specific aggregation sites to find a mate. The most noteworthy of all aggregation phenomena is “hilltopping”, where males of certain tachinid species fly to the tops of hills or mountains to await the arrival of females. Each species has its own predetermined time of day during which it will hilltop, with some species preferring the morning, others the afternoon, and the night-active Ormia hilltopping at dusk. On hot days the activity begins earlier than on cool days, and on cloudy days there are fewer flies. A good hilltop on a hot and sunny day is a frantic place, with anywhere from a few tachinid species to 75 or more (in exceptional habitats) visiting the hilltop by mid afternoon, after which fly activity drops off considerably. Virtually all the observable individuals are males, presumably because they remain on the hilltop for some time whereas females stay only long enough to find a mate. Males of each species are guided by instinct to a particular place on a hilltop and successive generations will return to the same spot year after year. One species may prefer a sunlit spot on the ground at the base of a bush, another will alight on tree trunks, and others will sit on foliage to one side of the hilltop or buzz around the topmost branches of a bush at the hilltop’s center, etc. Males of some species spend most of their time perched at preferred locations, darting out frequently to inspect passing insects. Males of other species are in almost constant motion as they fly from point to point but seldom alight.
Aggregation sites for male tachinids are not always on hilltops. The branches of an isolated tree, or shrubs alongside a stream, or a patch of ground below a bush, are a few of the places where males of one or more species may congregate to await a potential mate, nearly always in sunlit situations. Tree trunks also serve as aggregation sites for a number of species. Often several males will occupy the same tree trunk, but the males of some species (e.g., Trixodes obesus) are always found singly. Generally only one or a few trees in an area are attractive to aggregating males and these trees will be frequented by the same species year after year. Some species do not alight on a tree but instead fly in a zig-zag pattern up the trunk for a few moments before flying to another tree to repeat the behavior.
It is not uncommon for the antennae of male tachinids to be larger than those of females, but usually this difference is slight. Rarely, the difference is dramatic with the first flagellomere greatly enlarged over that in its conspecific female, or even bifid or multi-branched (Fig.2). Since antennae serve a mostly olfactory function, it is presumed that such antennae help in the location and/or courtship of females. Very little is known about courtship and mating in tachinids, especially under natural conditions.
Ecology
The vast majority of hosts of tachinid flies are plant-feeding insects. As a result, parasitism by tachinids has two major effects at the community level: a reduction of host populations, and an indirect reduction in feeding damage to plants used by hosts. The level of parasitism can vary greatly, from less than 1% to approaching 100%, depending on such factors as the size of a host population, size of the parasitoid population, and environmental conditions. Parasitism levels usually vary locally from one portion of a host’s range to another. Parasitism rates are quite dynamic and rise and fall through the generations and from area to area in cycles that both influence, and are influenced by, host populations. The net effect is generally low rates of parasitism (under 5%) with occasional generations where parasitism is significantly higher. Most of the studies into parasitism rates by tachinid flies have involved pest insects, some of which cause damage to crops or forests on an annual basis and some of which are only of economic concern during outbreaks. How quickly a tachinid population can take advantage of an increase in host numbers is dependent on a number of factors but is influenced in part by the oviposition strategy and fecundity of the tachinid species involved.Tachinid species can be broadly divided into two types, specialists and generalists. As the names imply, specialists attack one or a few host species and generalists attack multiple hosts. On the whole, tachinids tend to be less host specific than hymenopteran parasitoids. Factors affecting tachinid host range include, but are not limited to, habitat choice, host searching strategy, oviposition strategy, method for coping with physiological defenses of host, and developmental synchrony. There is recent evidence from large scale, long term, rearings of Tachinidae from Lepidoptera in Costa Rica that some supposedly generalist tachinid species actually comprise a group of specialist cryptic species (species that are difficult to separate morphologically). Although it is likely true that cryptic species are more common in the Tachinidae than previously thought and these species may have narrow host ranges, there are still many tachinids species that are clearly generalists. Most generalists have fewer than 20 recorded hosts, but some have over 50 and a very few have over 100. The hosts of many tachinid species are either unknown or poorly known and are not completely known for the vast majority of species.
Tachinid flies and other parasitoids exert selection pressures on their hosts to evolve ways in which to minimize parasitism. The more obvious may involve the evolution of cryptic coloration, specialized feeding strategies such as concealed feeding or feeding at night, or evasive maneuvers that are evoked when a host is attacked. Less obvious, but quite important at the community level, are the effects of tachinid parasitism on host and host-plant interactions (i.e., tritrophic interactions). Insect herbivores do not always feed preferentially on plant species that provide the highest nutrition. Certain insect herbivores will feed on less preferred host plants if such feeding reduces their level of mortality due to parasitism. The investigation of enemy-free space as an important component of tritrophic interactions is an active area of research.
Biological control
Potential insect pests are kept largely in check by their natural enemies in their native habitats, but the spread of agriculture and the introduction of insects into new areas has resulted in a steady increase in the number of economically important insect pests attacking crops and forests. Control measures continue to be largely chemical-based, but the benefits of biological control or integrated pest control (control by chemical, biological and other means) have long been recognized. By the early 1900s there was an increasing realization that some insects unwittingly transported to new places by sea vessels were causing considerable damage in their new locations. An early example concerns the gypsy moth (Lymantria dispar) and browntail moth (Euproctis chrysorrhoea), two forest pests introduced into eastern North America from Europe prior to 1900. In one of the earliest cases of a classical biological control program, several tachinid parasitoids of these pests were imported into eastern USA from Europe in huge numbers in the early 1900s. Although the program was not a complete success, it laid the groundwork for future studies of tachinids as biological control agents. In recent years, one of the tachinid species introduced against those forest pests, Compsilura concinnata, has been implicated in the decline of giant silk moths (Saturniidae) in northeastern United States.
Some of the best known success stories of tachinid biological control programs have been reviewed many times. They include: the release of Cyzenis albicans from Europe against the winter moth (Operophtera brumata) in Canada, release of Lixophaga diatraeae from Cuba and elsewhere and Lydella minense from Brazil against sugarcane borers (Diatraea spp.) in the Caribbean, release of Bessa remota from Malaysia against the coconut moth (Levuana iridescens) in Fiji, and release of Ceromasia sphenophori from Papua New Guinea against the New Guinea sugarcane weevil (Rhabdoscelus obscurus). More recently, Ormia depleta from Brazil has been implicated in the control of pest mole crickets (Scapteriscus spp.) in Florida, USA. Similarly, several species of Trichopoda from the southern United States and South America have been introduced recently into Hawaii, Australia, Italy (accidentally), South Africa, and other places for control of the southern green stink bug (Nezara viridula), with mixed results.
Some tachinid species have been successfully mass-produced for biological control programs whereas others have proved difficult or expensive to rear under laboratory conditions. The in vitro propagation of tachinids on artificial diets has been achieved for a very few species in laboratory experiments but has yet to be employed in a biological control program.
Acknowledgements
I thank Shannon Henderson (Agriculture & Agri-Food Canada, Ottawa) for photographing the specimens shown here. All specimens belong to the Canadian National Collection of Insects (Ottawa).
References
Belshaw, R. 1994. Life history characteristics of Tachinidae (Diptera) and their effect on polyphagy. Pp. 145–162. In: Hawkins, B.A. and Sheehan, W., eds., Parasitoid community ecology. Oxford University Press, Oxford. 516 pp.
Grenier, S. 1988. Applied biological control with tachinid flies (Diptera, Tachinidae): a review. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz 61: 49–56.
O’Hara, J.E. 2008. World genera of the Tachinidae (Diptera) and their regional occurrence. Version 4. PDF document, 71 pp. Published on the Internet at http://www.nadsdiptera.org/Tach/Genera/generahom.htm
Stireman, J.O., O’Hara, J.E. and Wood, D.M. 2006. Tachinidae: evolution, behavior, and ecology. Annual Review of Entomology 51: 525–555.
Tschorsnig, H.-P. and Richter, V.A. 1998. Family Tachinidae. Pp. 691–827. In: Papp, L. and Darvas, B., eds., Contributions to a Manual of Palaearctic Diptera (with special reference to flies of economic importance). Volume 3. Higher Brachycera. Science Herald, Budapest. 880 pp.
Wood, D.M. 1987. Tachinidae. Pp. 1193–1269. In: McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R. and Wood, D.M., eds., Manual of Nearctic Diptera. Volume 2. Agriculture Canada Monograph 28: i–vi, 675–1332.

